what types of atoms compose each type of compound
Up to 24 cash back What types of atoms compose each type of compound only metals only nonmetals or both. For atoms with differing electronegativity the bond will be a polar covalent interaction where the electrons will not be shared equally.
 |
| Key Introduction To Ionic Covalent Bonding |
2 Get Another question on Chemistry.
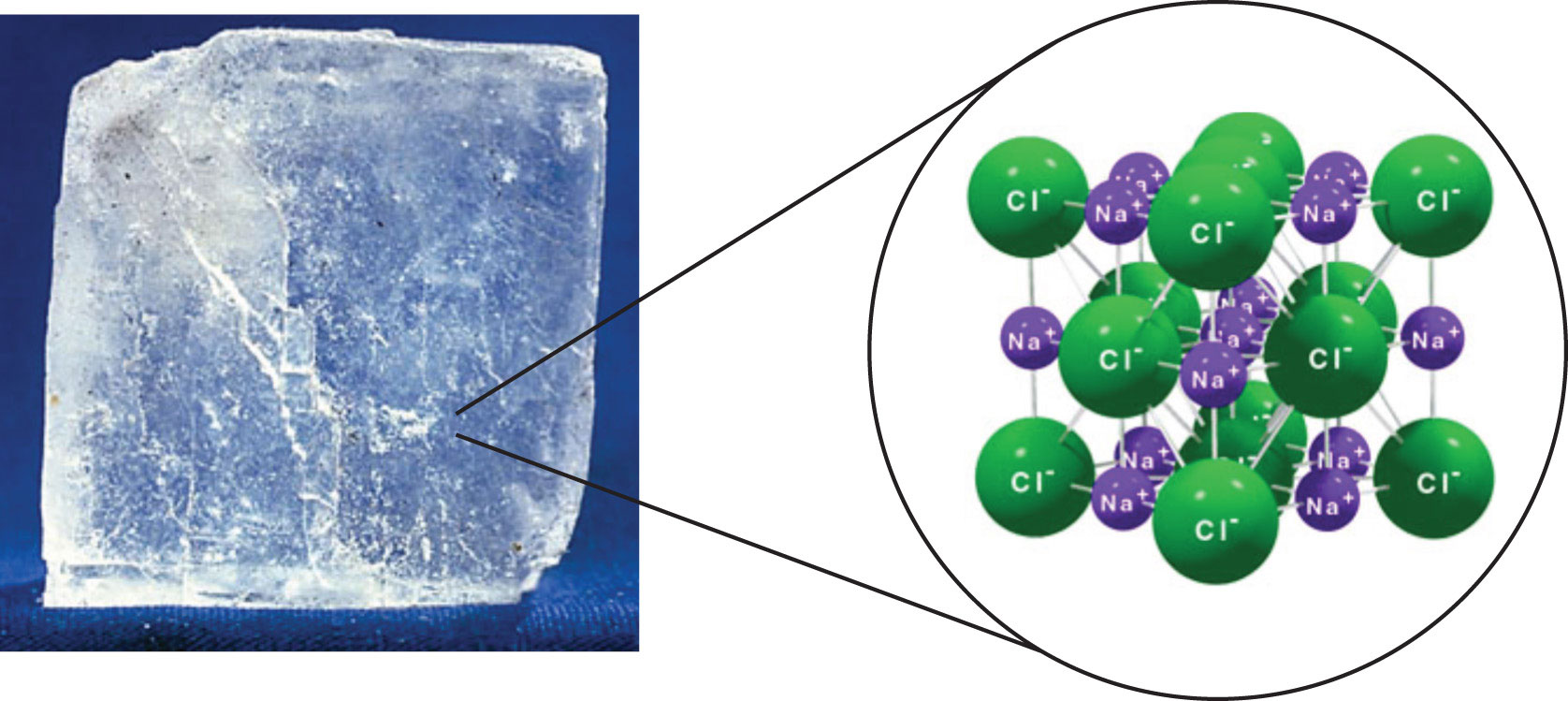
. What types of atoms compose each type of compound only metals only nonmetals or both. Pure air Solution uniform physical combination of gases such as nitrogen oxygen carbon dioxide and argon. Procedure Part A Macro Tab Open the Sugar and Salt Solutions simulation on the PhET website by following the link below and clicking Run Now-salt-solutions Drag the conductivity tester labeled A into the. CH 3 Molecular formula.
Covalent compounds on the other hand have lower melting and boiling points. Tertiary Carbon 3 Carbon attached to three other carbons. In molecular compounds the atom binds each other through covalent bonds. In salts it is held together with ionic bonds.
Only nonmetals or both. That means that sodium will also have 11 electrons and chlorine will also have 17 electrons. There are two aluminum atoms there are three carbon atoms there are nine oxygen atoms. How many of each type of ion and each type of atom occurs in the smallest identifiable unit of the ionic compound Al 2 CO 3 3.
Sodium has 11 protons and chlorine has 17. There are two types of bonds ionic and covalent. What types of atoms compose each type of compound only metals in progress 0 Physics Bình An 6 months 2021-07-31T2016130000 2021-07. You could see waters chemical formula it says it has 2 atoms of.
A chemical formula tells us the number of atoms of each element in a compound. Example of compounds includes water H 2 O Hydrogen Peroxide H 2 O 2 etc. Express your answer to four significant figures. What types of atoms compose each type of compound only metals only nonmetals or both.
The molecular formula gives the exact number of each type of atom in the molecule. There are two aluminum ions Al 3 there are three carbonate ions CO 3 2---within each carbonate ion there is one carbon and three oxygens. Sodium iodide NaI d. Heteronuclear Diatomic Molecules -- A heteronuclear diatomic molecule consists of two of atoms of the same element combined.
Type of Bond potassium chloride KCl solid Yes Yes benzoic acid C 6 H 5. Of a letter figure or symbol written or printed below the line. For example water H 2 O has three atoms two hydrogen H atoms and one oxygen O atom. Hydrogen H 2 Nitrogen N 2 Oxygen O 2 Fluorine F 2 Chlorine Cl 2 --Iodine I 2 and Bromine Br 2.
These are the two types of bonds out of which every compound is made of. Secondary Carbon 2 Carbon attached to two other carbons. When metals and non metals react whichs atoms gain electrons. Leave a Reply Cancel reply.
The three main types of bonds are ionic covalent and metallic. Ionic solids are generally characterized by high melting and boiling points along with brittle crystalline structures. Compounds are made of two or more atoms of different elements such as water H 2 O and methane CH 4. Based on the formula predict whether each of the following compounds is primarily ionic or primarily covalent.
For example in Figure 123 the molecular formula of 2-methyl propane is C 4 H 10. Here are two atoms sodium and chlorine. In an ionic bond which is a compound one of the atoms donates one or more electrons to another atom to make both of their valence shells full. A substance that contains two or more elements chemically combined a fixed proportion.
For example most samples of the elements hydrogen oxygen and nitrogen are composed of molecules that contain two atoms each called diatomic molecules and thus have the molecular formulas H 2 O 2 and N 2 respectively. Types of Chemical Compounds. Carbon dioxide Compound uniform matter composed of two types of atoms C O chemically united. Required fields are marked.
Compound uniform matter composed of two types of atoms H O chemically united. Which of the following options correctly describe each type of chemical compound. What types of atoms compose each type of compound - only metals only nonmetals or both. It contains the symbols of the.
Your email address will not be published. The molecular formula is a concise way of expressing information about the atoms that make up a particular covalent molecular compound. -compounds contain two or more different types of atoms-an atom is the smallest unit of an element that can exist as a stable independent entity. There are seven diatomiceElements.
Calculate the molar mass of aluminum oxide al2o3. -Composed of molecules- molecular. Atoms of more than one type of element can bond together and form compounds. Atoms Molecules and Ions Types of Formulas Empirical formulas give the lowest whole-number ratio of atoms of each element in a compound.
It a molecular compound because the hydrogen and oxygen atoms are both nonmetals and a molecular compound consists of nonmetals. An atom or group of atoms that has a positive or negative charge. The types of atoms compose each type of compound if only metals then it is Covalent bond. Pre-Lab Questions Define ionic bond.
A compound is a substance made up of a definite proportion of two or more elements. Atoms are not drawn to scale. It is regarded as a chemical bond that entails sharing a pair of electrons between atoms in a. The classifications are as follow.
What types of atoms compose each type of compound only metals only nonmetals or both. These distinct types of. Molecules of compounds have atoms of two or more different elements. Covalent bond is when an atom will form a bond by sharing electrons.
Molecular formulas give the exact number of atoms of each element in a compound. Primary Carbon 1 Carbon attached to one other carbon. If both then it is ionic bond that is composed of both metal and nonmetal. Define covalent bond.
For reasons that are beyond the scope of this post sodium wants to lose an electron and chlorine wants to gain an electron.
 |
| Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube |
 |
| Elements Compounds And Mixtures Compounds And Mixtures Elements Compounds And Mixtures Element |
 |
| Chemical Bonding Doodle Notes Science Doodle Notes Doodle Notes Science Doodle Notes Science Doodles |
 |
| Carbon Chemistry Carbon Atoms Can Form Single Double Or Triple Bonds With Other Carbon Atoms Carbon Can Form Up To 4 Bonds This A Atom Carbon Cycle Chemistry |
 |
| Molecules And Compounds Overview Atomic Structure Article Khan Academy |
Posting Komentar untuk "what types of atoms compose each type of compound"